Table of Contents
Acetic Acid: The Ultimate Multitasker with a Comedic Flair
Introduction:
Acetic acid (CH3COOH), also known as ethanoic acid, is like the MacGyver of organic compounds – versatile, ever-present, and crucial to many of our daily needs. This carboxylic acid comprises a methyl group shaking hands with a carboxyl functional group, which is the superstar behind vinegar (dilute acetic acid solution), the ultimate condiment. With a vast number of uses, acetic acid moonlights as a food preservative, industrial solvent, and medicinal marvel, among other roles.
Structure of Acetic Acid:
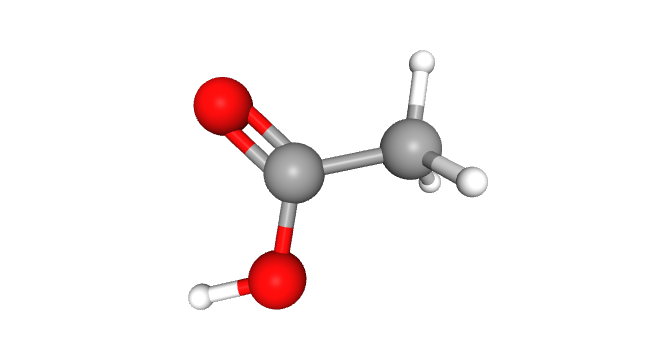
Acetic acid’s structure is best represented by CH3(C=O)OH, or CH3CO2H. It’s like the younger sibling of formic acid (HCOOH), the simplest carboxylic acid. In its solid state, acetic acid molecules act like a group of friends, forming chains and bonding via hydrogen bonds. Even as a liquid, this party doesn’t stop – acetic acid dimers can still be found in dilute solutions, though they’re somewhat shy around solvents that promote hydrogen bonding.
Physical Properties of Acetic Acid:
Acetic acid’s sour personality is evident as a colorless liquid with a pungent, nose-tickling smell and a mouth-puckering taste. This corrosive liquid is completely miscible with water, and at standard temperature and pressure (STP), it has melting and boiling points of 16.6 °C (61.9 °F) and 117.9 °C (244.2 °F), respectively. With a molar mass of 60.052 g/mol and a density of 1.05 g/cm³, acetic acid is the party’s life in its liquid form.
Production of Acetic Acid:
Acetic acid owes its existence to a process developed in the 1960s by the chemical company Monsanto. This three-step dance, involving a rhodium-iodine catalyzed carbonylation of methanol (methyl alcohol), can be summarized as follows:
- CH3OH (methanol) + HI (hydrogen iodide) → CH3I (methyl iodide intermediate) + H2O
- CH3I + CO (carbon monoxide) → CH3COI (acetyl iodide)
- CH3COI + H2O → CH3COOH (acetic acid) + HI
Other ways of preparing acetic acid include the oxidation of acetaldehyde, ethylene oxidation with a palladium catalyst and heteropoly acid, and direct conversion of sugar into acetic acid by some anaerobic bacteria (talk about multitasking!).
Chemical Properties of Acetic Acid:
Like other carboxylic acids, Acetic acid participates in chemical reactions. When heated above 440 °C, it decomposes into either methane and carbon dioxide or water and ethenone. Certain metals, such as magnesium, zinc, and iron, react with acetic acid, forming acetate salts and leaving behind the scent of a chemistry lab.
Uses of Acetic Acid:
Acetic acid is an actual Renaissance compound, finding uses in various industries:
- As an antiseptic, it shows off its antibacterial qualities.
- It has a hand in manufacturing rayon fiber, rubber, and delightful perfumes.
- Acetic acid is a cancer-fighting superhero injected directly into tumors.
- It’s the secret ingredient in pickling vegetables, courtesy of its presence in vinegar.
- Acetic acid is a crucial player in producing vinyl acetate monomer (VAM), which is used in manufacturing paints and adhesives. VAM is essential for producing polyvinyl acetate (PVA) and polyvinyl alcohol (PVOH), which are widely used in adhesives, paints, and coatings.
- The textile industry loves acetic acid because it helps dyes bind to fibers and increases colorfastness, resulting in longer-lasting and more vibrant colors on fabrics. Fashionistas everywhere rejoice!
- The food industry’s best friend is acetic acid, acting as an acidulant, preservative, and flavor enhancer. It plays a starring role in pickling vegetables, inhibiting bacterial growth, and maintaining freshness and taste. It’s also a key ingredient in condiments like mayonnaise, mustard, and ketchup, as well as in salad dressings and baked goods.
- As a solvent, acetic acid is popular in various industries, including manufacturing inks, paints, and coatings. Its ability to mingle with polar and nonpolar compounds makes it a versatile and essential industrial solvent. It’s also a crucial component in the industrial preparation of dimethyl terephthalate (DMT).
- In the cleaning industry, acetic acid moonlights as a descaling agent, removing mineral deposits from surfaces and dissolving organic compounds like sulfur and iodine. It’s used in the production of household cleaning products, where its antibacterial properties make it an effective disinfectant.
- Acetic acid shines in the medical field with its antibacterial qualities. As an antiseptic, it is effective against various bacteria, including pseudomonas, enterococci, streptococci, and staphylococci. Moreover, acetic acid is used in the detection of cervical cancer and the treatment of infections. In some cases, it has even been directly injected into tumors as a cancer treatment.
In conclusion, acetic acid is an essential organic compound with a wide range of applications across various industries. Its unique chemical and physical properties make it a key component in the production of everyday products, such as textiles, plastics, and cleaning agents. Additionally, its antibacterial and preservative qualities make it a vital ingredient in the food and pharmaceutical industries. From industrial uses to household applications, acetic acid plays a significant role in our daily lives, proving it truly is the MacGyver of organic compounds.
Let’s Talk Acetic Acid: A Humorous and Knowledgeable FAQ
Q1: What’s the deal with acetic acid? What’s it good for? Well, my friend, acetic acid is the life of the vinegar party! It’s the main ingredient that gives vinegar its signature tangy taste. But wait, there’s more! Acetic acid moonlights are an essential part of vinyl acetate monomer (or VAM, for those in the know), which stars in creating fabulous paints and super-sticky adhesives. Just think of acetic acid as the superhero of the chemical world!
Q2: Is acetic acid like a super-strong acid? No, not really. Acetic acid (CH3COOH) is more of a chill, a weak acid. When hanging out with a strong base, it only gets its groove with complete dissociation. Hydrochloric acid, conversely, is the genuine muscle-bound tough guy in the acid world.
Q3: How does one whip up a batch of acetic acid? You’ll need methanol, hydrogen iodide, and carbon monoxide to cook up some acetic acid:
- Mix the methanol and hydrogen iodide for a scrumptious methyl iodide concoction.
- Toss in some carbon monoxide to create acetyl iodide.
- Please give it a good hydrolysis shower, and voilà!
You’ve got yourself some acetic acid.
Q4: So, is acetic acid basically vinegar? Well, not exactly. Vinegar is more like acetic acid’s less-concentrated, mild-mannered cousin. It’s an acetic acid solution in the water, containing about 5% to 8% ethanoic acid by volume. But don’t be fooled by its mild demeanor; it still packs a flavorful punch!
Q5: Is acetic acid a danger to us mere mortals? You need to treat it with respect. You will have a wrong time if you go sniffing around concentrated acetic acid solutions (we’re talking >25% here). Breathing in those gnarly vapors can cause a whole lot of unpleasantness, like eye, nose, and throat irritation, coughing, chest tightness, headaches, fever, and even confusion. So, remember, kids, play it safe and keep your acetic acid concentrations on the lower side!
Check Our Chemistry Blog Here: Unravel the Secrets of Chemistry with Engaging Articles
Click Here to Dive Back into Our Educational Resources and Expand Your Knowledge Blog