Table of Contents
Acetone: From Earth to Space and Everything in Between
Intro:
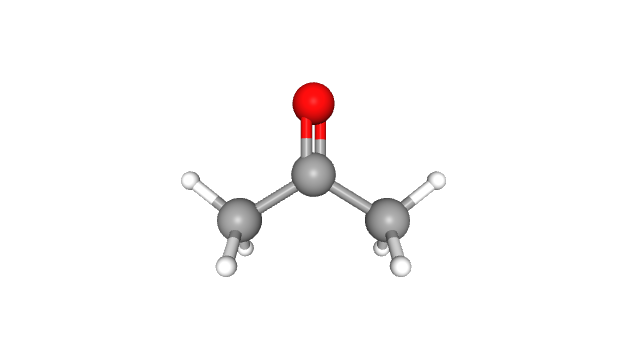
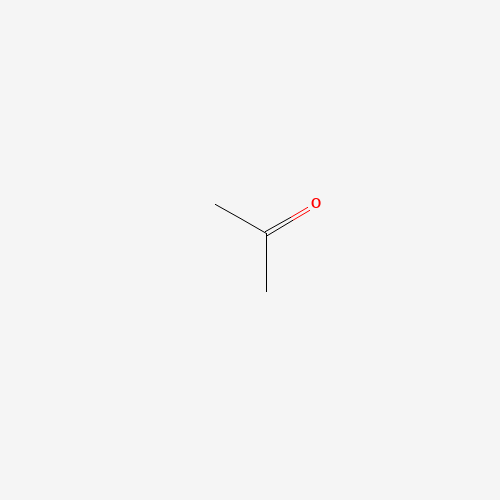
Acetone, the tiny yet mighty molecule, also goes by the names dimethyl ketone and propanone. This aliphatic (fat-derived) ketone packs a punch as the smallest and most important of its kind. Donning the appearance of a colourless, fruity-scented liquid, acetone is volatile and flammable. It might remind you of nail polish remover, but this molecule has an impressive resume that goes beyond just cosmetics. So, let’s dive into the acetone universe, filled with fun facts, entertaining examples, and a splash of humour!
The Amazing Acetone Attributes
Acetone is the life of the party, mingling easily with substances like water, ethanol, and ether. Its miscibility makes it an all-star solvent and easily integrates with water.
With a freezing point of -94.7 °C, acetone could challenge Elsa from “Frozen” in a sub-zero showdown!
Acetone’s boiling point reaches 56.05 °C, with a flash point of -20 °C. Its eagerness to evaporate rivals that of a genie itching to escape its lamp! This low flash point makes acetone a bit of a firestarter, so it’s often stored in metal drums to prevent static charges from igniting it.
Like the Swiss Army knife of solvents, acetone can dissolve fats, resins, cellulose acetate, and even nitrocellulose. It’s practically a “Universal Solvent” in the making.
Acetone’s Adventures in the Natural World
Acetone loves to socialize and can be found as:
Hobnobbing with plants, trees, volcanic gases, and forest fires.
Breaking down body fat in a process called ketosis means we all have a little acetone party going on inside of us! This can lead to higher acetone concentrations in the blood and urine of people with diabetes, pregnant women, breastfeeding mothers, and children.
Showing up in small quantities in normal urine and blood, and in higher concentrations in diabetic patients.
Hanging out in vehicle exhaust, tobacco smoke, and landfill sites.
The Tale of Acetone: From Alchemy to Chemistry
Acetone’s journey began in the hands of alchemists during the late Middle Ages, who dubbed it the “spirit of Saturn.”
In 1832, French chemist Jean-Baptise Dumas and German chemist Justus von Liebig figured out acetone’s empirical formula.
Antoine Bussy, another French chemist, named acetone in 1833 by adding the suffix -one to the stem of the corresponding acid, acetic acid.
Chaim Weizmann developed the Weizmann Process for industrial acetone production during World War I, which proved invaluable for Britain’s war effort to produce cordite, a military-grade explosive.
The Acetone Assembly Line: From Cumene to Wacker-Hoechst
About 83% of acetone production comes from the cumene process.
The United States leads in acetone production capacity, followed by Taiwan and China. INEOS Phenol is the largest acetone producer, owning 17% of the world’s capacity.
The Many Hats of Acetone: A Versatile Virtuoso
Solvent: Acetone’s social skills make it an excellent solvent for plastics, synthetic fibres, paints, and varnishes. Laboratories also rely on it for cleaning.
Chemical Intermediate: Acetone helps create compounds like methyl methacrylate and bisphenol A.
Laboratory Use: As a polar aprotic solvent, acetone plays a part in various organic reactions and is commonly used as a rinsing agent for laboratory glassware.
Medical and Cosmetic Uses: Acetone is a popular ingredient in generic medicines, cosmetic products, and food additives. The American Food & Drug Administration labels it ‘generally recognized as safe’ (GRAS), making it safe for use in small quantities in food and cosmetics.
Other Domestic Uses: Acetone is a key component of nail polish, paint thinners, and superglue remover.
Acetone in Space: The Final Frontier
In a surprising interstellar twist, acetone was discovered on the surface of comet 67P during Philae lander’s first touchdown in 2015. Four of the 16 organic compounds detected, including acetone, were new to comets. Acetone’s presence isn’t limited to Earth; it’s also found in the far reaches of space!
Conclusion: Acetone, the Mighty Mini Molecule
Acetone’s fascinating history, diverse uses, and extraterrestrial presence make it a remarkable compound. Acetone is a versatile and indispensable molecule from its modest beginnings with alchemists to its crucial role in industries, laboratories, and households. As we continue to unravel the mysteries of acetone and its applications, we celebrate the wonders of this seemingly simple yet extraordinary molecule. Cheers to acetone—the little molecule with big ambitions!
Check Our Chemistry Blog Here: Unravel the Secrets of Chemistry with Engaging Articles
Click Here to Dive Back into Our Educational Resources and Expand Your Knowledge Blog