Table of Contents
Introduction:
Acid and base are fascinating chemicals that play significant roles in our daily lives. From cleaning products to food and household items, these substances are ubiquitous. This article will delve into the definitions, properties, differences, and applications of acids and bases, exploring the theories underpinning their behaviour.
Definition and Properties of Acids
If a substance dissolves in water and releases hydrogen ions (H+), it is classified as an acid. Moreover, acids can either donate protons (H+) or accept electrons in chemical reactions. Acids are identifiable by their unique characteristics, including:
- Conducting electricity: Acids are conductive due to the presence of ions in their solution. This property makes them essential in various electronic applications.
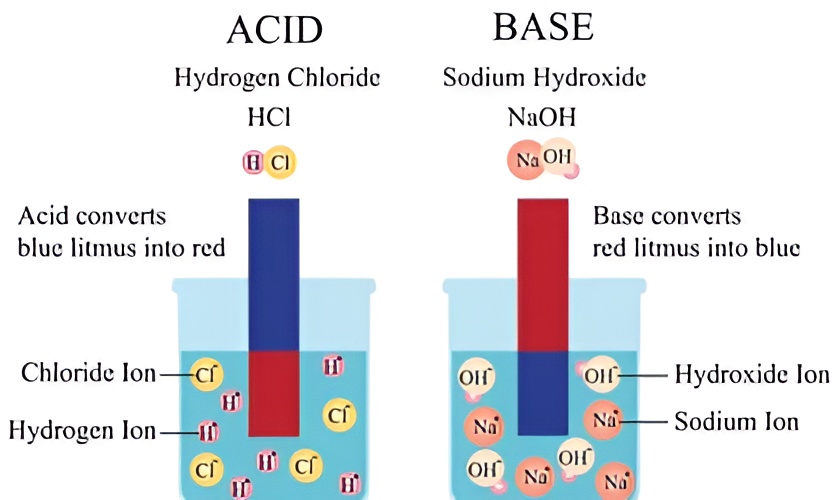
- Turning blue litmus paper red: One classic test for acids involves placing a blue litmus paper in their presence, which will change colour to red. This simple demonstration helps identify the presence of an acid.
- Sour taste: Acids often exhibit a sour taste, although it’s worth noting that tasting or ingesting solid acids can be extremely dangerous and should be avoided.
- Reacting with metals to produce hydrogen: In experiments carried out in classrooms, when acids come into contact with specific metals like zinc or magnesium, they cause a chemical reaction that releases hydrogen gas.
- pH value below 7: The pH scale measures whether a substance is acidic or alkaline. It ranges from 0 to 14. A pH value below 7 indicates acidity, while a value of 7 is neutral.
Acids can be further classified as strong or weak. Strong acids, like hydrochloric acid (HCl) and nitric acid (HNO3), fully dissociate into ions when mixed with water. In contrast, when mixed with water, weak acids like hydrofluoric acid only dissociate partially into ions.
Definition and Properties of Bases
Bases are substances that can donate electrons, accept protons, or release hydroxide (OH-) ions in water. They also exhibit intriguing properties, including:
- Slippery texture: When you touch a base, you might notice a slippery sensation, similar to how the soap feels. This characteristic arises from the reaction between bases and oils on our skin.
- Bitter taste: Bases often have a bitter taste. However, just like with acids, avoiding tasting or consuming strong bases is crucial due to their potential harm.
- Turning red litmus paper blue: A distinct feature of bases is their ability to change the colour of red litmus paper to blue. This reaction serves as a quick test for determining the presence of a base.
- pH value above 7: Bases have a pH value above seven on the pH scale, indicating their alkaline nature. Substances that have a pH value of 7 are classified as neutral.
- Good conductors of electricity in aqueous solutions: Bases, similar to acids, conduct electricity when dissolved in water. This property contributes to their applications in various industries.
Bases can be classified as strong, weak, neutral, super, or solid bases. When mixed with water, sodium hydroxide (NaOH) and potassium hydroxide (KOH) dissociate entirely into ions. These are considered strong bases. Conversely, weak bases do not fully dissociate and exist in equilibrium with their ions.
Differences Between Acid and Base
Acid and base possess fundamental differences that distinguish their behaviour and characteristics. Some notable distinctions include:
- Ion release: When acids are mixed with water, they release hydrogen ions (H+), while bases release hydroxide ions (OH-).
2. pH value: Acids are characterized by a pH value below 7, indicating acidity and bases are characterized by a pH value above 7, indicating alkalinity.
3. Litmus paper test: When blue litmus paper comes into contact with acids, it turns red. On the other hand, when red litmus paper is exposed to bases, it turns blue. This test can help you quickly and easily differentiate between acids and bases.
4 . Chemical formulas: Acids typically start with the chemical formula H, such as HCl for hydrochloric acid, while bases have the chemical formula OH at the end, like NaOH for sodium hydroxide.
Theories Defining Acid and Base
To further understand the behaviour of acid and base, scientists have developed three main theories:
- Arrhenius theory: According to Svante Arrhenius, acids produce hydrogen ions (H+) in an aqueous solution, while bases produce hydroxide ions (OH-). This theory provides a foundational understanding of acid-base reactions and their ionization.
- Bronsted-Lowry theory: Proposed by Johannes Nicolaus Bronsted and Thomas Martin Lowry, this theory defines acids as proton donors and bases as proton acceptors. In this concept, acids transfer protons to bases during chemical reactions. It broadens the definition of acids and bases beyond aqueous solutions, encompassing reactions in various contexts.
- Lewis theory(lewis acid and base): Developed by Gilbert N. Lewis, the Lewis theory defines acids as electron-pair acceptors and bases as electron-pair donors. This theory expands the understanding of acids and bases beyond proton transfer, acknowledging the role of electron pairs in chemical reactions.
Uses of Acid and Base
Acid and base find extensive applications in both everyday life and industrial processes. Some notable uses include:
Acids:
- Food preservation: Acids like citric acid (found in citrus fruits) and vinegar are commonly used for preserving food due to their antimicrobial properties.
- Batteries: Sulfuric acid plays a crucial role in the functioning of lead-acid batteries, commonly used in vehicles and backup power systems.
- Industrial production: Acids such as sulfuric acid and nitric acid are essential in the manufacturing processes of explosives, dyes, paints, and fertilizers.
Bases:
- Soap and detergent production: Sodium hydroxide and potassium hydroxide, commonly known as lye, are vital ingredients in producing soaps and detergents.
- Antacids: Bases like magnesium hydroxide are used in antacid medications to neutralize excess stomach acid and alleviate heartburn and indigestion.
- Laboratory reagents: Ammonium hydroxide is commonly used as a reagent in laboratory settings for various chemical reactions and analyses.
- Soil treatment: Bases such as slaked lime (calcium hydroxide) are used in agriculture to modify soil pH and improve its fertility.
Conclusion
Acid and base are essential substances with distinct properties, behaviours, and applications. By understanding their definitions, properties, differences, and the theories that define them, we gain insight into their roles in various chemical reactions and everyday life. Whether it’s the tangy taste of an acid or the slippery texture of a base, these compounds leave a lasting impression on our experiences and interactions with the world around us. So, let’s embrace the fascinating world of acids and bases and appreciate the chemistry they bring to our lives, minus any unexpected bubbly reactions in our morning coffee!
Check Our Chemistry Blog Here: Unravel the Secrets of Chemistry with Engaging Articles
Click Here to Dive Back into Our Educational Resources and Expand Your Knowledge Blog